Forgotten digital tourniquets leading to digit amputation is the single greatest risk posed by their use. Covering the digit in gauze before a patient is discharged can hide an inconspicuous tourniquet. Our most persistent competitor in the marketplace are improvised methods like using rigged surgical gloves or other elastic materials, which lack great visibility unlike Mar-Med’s Tourni-Cot and Uni-Cot.
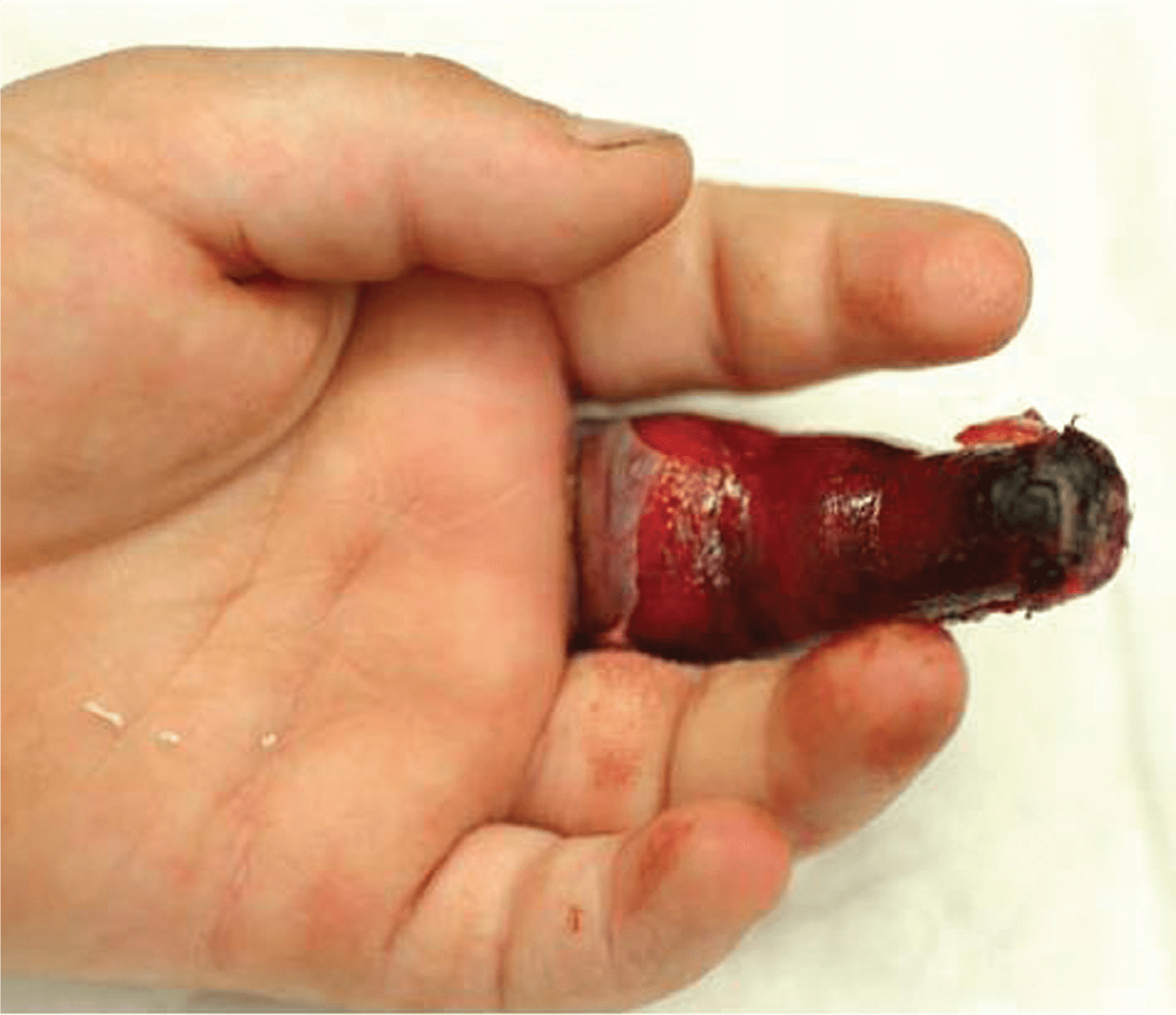
In 2009 the United Kingdom’s National Patient Safety Agency (NPSA) came out with a Rapid Response Report entitled “Reducing risks of tourniquets left on after finger and toe surgery”. The report documents a total of 15 relevant cases recorded from 2005-2009. A surgical glove was used in 6 of the cases and 2 of the cases resulted in digit amputation. While the report acknowledges that the population affected is relatively small, it also cites the great degree of harm and high cost of litigation.
The report goes on to say in part that “purpose designed visible tourniquets are available and are intended for this use. Data from reported incidents suggest that at least some of the preventable harm is caused by the use of surgical gloves.”
The NHS report identifies some key actions that can be taken toward safer practice, including:
- CE marked digital tourniquets which are labelled and/or brightly coloured should be used, in accordance with manufacturers’ instructions. Surgical gloves should not be used as tourniquets.
- Guidelines include the removal of digital tourniquets as part of the swab counting procedure and the need to record the length of time a tourniquet is in place.
- The World Health Organization (WHO) Surgical Safety Checklist is reviewed locally to consider adding tourniquet removal at ‘Sign Out’ stage.
Mar-Med’s digital tourniquets, the Tourni-Cot and Uni-Cot, are both brightly colored to be sure they are highly visible. The Tourni-Cot comes with a caution tag attached making it almost impossible to forget (clearly marked “Do Not Remove This Tag”). The Uni-Cot was designed with large handles that increase visibility. Both Mar-Med devices are CE marked, indicating compliance with current European legislation of goods (the French phrase “Conformité Européene” translates to “European Conformity”.)


The UK’s NHS must have had Mar-Med in mind for the Rapid Response Report. Clinicians in the UK and Europe have enjoyed the Tourni-Cot for years before the report was published, without other digital tourniquets on the market. Some clinicians I speak with will comment that removing the Tourni-Cot’s caution tag can make it less conspicuous. To that I respond by echoing the UK guidelines to follow our instructions and simply “Do Not Remove This Tag”.
External Links: Reducing Risks of Tourniquets Left on After Finger and Toe Surgery, UK NHS National Patient Safety Agency.